Stoichiometry Mass to Mass Worksheet Answers: Unveiling the Secrets of Chemical Proportions. Dive into the captivating world of stoichiometry, where the precise dance of chemical elements unfolds. With our comprehensive guide, conquer mass-to-mass worksheet challenges, unravel the mysteries of chemical equations, and witness the transformative power of stoichiometry in real-world applications.
In this comprehensive guide, we embark on a journey through the intricacies of stoichiometry, empowering you with the knowledge and skills to navigate mass-to-mass worksheet problems with precision. Master the art of balancing chemical equations, seamlessly convert between moles and grams, and harness stoichiometry to accurately predict the quantities of reactants and products in any chemical reaction.
Stoichiometry Mass to Mass Worksheet Basics
Stoichiometry adalah studi tentang hubungan kuantitatif antara reaktan dan produk dalam reaksi kimia. Mass-to-mass worksheet adalah alat yang digunakan untuk menghitung massa reaktan dan produk dalam reaksi kimia berdasarkan persamaan kimia yang diberikan.
Worksheet ini menyediakan tabel yang berisi informasi berikut:
- Persamaan kimia yang tidak seimbang
- Massa molar reaktan dan produk
- Kolom untuk menghitung mol reaktan dan produk
- Kolom untuk menghitung massa reaktan dan produk
Tujuan dari worksheet ini adalah untuk menyeimbangkan persamaan kimia, mengonversi antara mol dan gram, dan menggunakan stoikiometri untuk menghitung massa reaktan dan produk yang diperlukan atau dihasilkan dalam reaksi kimia.
Contoh:
Hitung massa natrium klorida (NaCl) yang dihasilkan ketika 5,0 g natrium (Na) bereaksi dengan 10,0 g klorin (Cl 2).
Persamaan kimia:
2 Na + Cl 2→ 2 NaCl
General Inquiries: Stoichiometry Mass To Mass Worksheet Answers
What is stoichiometry?
Stoichiometry is the branch of chemistry that deals with the quantitative relationships between reactants and products in chemical reactions.
How do I balance chemical equations?
To balance chemical equations, adjust the coefficients in front of each chemical formula until the number of atoms of each element is the same on both sides of the equation.
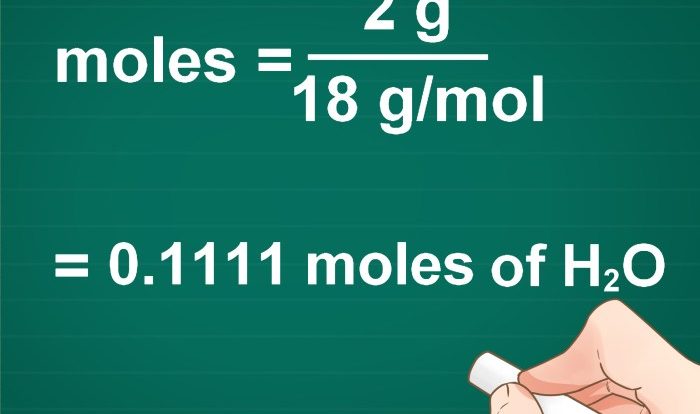
How do I convert between moles and grams?
To convert between moles and grams, use the molar mass of the substance. The molar mass is the mass of one mole of the substance in grams.